Water Purification Systems
Easy availability of laboratory pure water in required quantity and quality are fundamental conditions for the operation of laboratories. In the past, a common method of preparation of purified water - distillation is now almost replaced by a less energy-consuming and operationally reliable methods. The basis of most modern systems for the preparation of purified water by reverse osmosis, which in practice is combined with other principles such as adsorption, ion exchange, filtration, ultrafiltration, and other physical-chemical methods.
Reverze osmosis
The process of reverse osmosis (RO) as industrial water treatment method is used since the early 60 the 20 century. Originally, water treatment systems using the principle of reverse osmosis developed to demineralization of sea water. It soon became a key technology of drinking water in areas where freshwater resources are unavailable or limited in the coastal desert states. Using reverse osmosis systems are also indispensable in orbital stations, submarines, ocean-going ships. The method proved to be efficient and economical than any other water treatment methods such as distillation. Now frequently used in the preparation of water in laboratories, pharmaceutical manufacturing and the other applicatons. The usual efficiency of the reverse osmosis process is 90-99%.
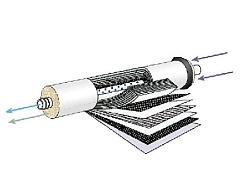
Filtration
Mechanical filters with given nominal porosity are the most common filters used to remove insoluble particules, and as a first step in facilities for the preparation of pure water. They are a necessary step of all the systems with reverse osmotic membrane, protecting it from irreversible blocking particles. Most are made by winding fibers on the central tube. As water passes through the filter into the central hole, the particles are trapped on the fibers. This type of filter removes most impurities above mentioned porosity filter. The term membrane ("absolute") filtration is used for filter process, where all particles are removed with a diameter equal to or greater than the porosity of the filter. These filters using a flat membrane or hollow fiber are usually included at the end of the system to remove bacteria and other particles not discarded by previous technologies. Traditionally used membrane filters with pore size below 0.47 micron, 0.2 micron frequently.

Ultrafiltration
Ultrafiltration membranes are primarily used in laboratory systems for ultrapure water to remove pyrogens (bacterial endotoxins), and as a tool to remove nucleases and DNA. Ultrafilters are based on combination of physical and chemical interactions. Implementation of ultrafiltration membranes in many ways reminds reverse osmotic membrane. Particles are trapped on the surface and rinsed in a special waste output. Ultrafilters are applied to the end of the system to ensure the almost hundred-percent removal of particles.
Adsorption
Adsorption as the cleaning process uses a large surface of activated carbon to remove organic compounds and free chlorine from incoming water. It is usually aplied as first or second stage in the purification process. Organic compounds and chlorine remain trapped on the surface of activated carbon and gradually deplete the capacity of the filter.

Deionization
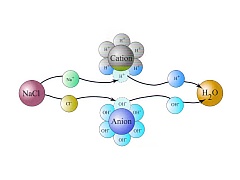
Deionization is also often referred as demineralization or ion exchange. In the process of deionization ions are removed from water using synthetic resins - ion exchangers. Ion exchangers have high affinity to dissolved inorganic substances of ionic nature. Depending on the type of ions that remove we distinguish cation (remove cations) and anion exchange resin (removing anions). Deionization is the only technology that is able to provide ultrapure water with resistivity up to 18.2 MOhm (which corresponds to a specific conductivity of 0.056 S / cm). In practice, most often used an equimolar mixture of very pure anion and cation exchanger (mixed-bed) allowing a maximum ionic purity. An important requirement for the ion exchangers used in the preparation of pure water is very minimal release of organic substances. A separate application is to remove hardness-causing cations (especially calcium and magnesium) using an cation exchanger in sodium cycle. The device is called softener and it is capable to perform automatic regeneration of cation exchanger using saturated sodium chloride solution.
Sterilization a photooxidation
Irradiation of water by ultraviolet "light" at a wavelength of 254 nm and 185 eliminates the traces of organic compounds and reliably kills microorganisms (UV paralyzes cellular metabolism). Radiation with a wavelength of 185 nm leads to the formation of ozone from dissolved oxygen and then the resulting free radicals oxidize organic compounds to the ionogenic molecules. They are then removed with a special anion exchanger with a very low release of organic substances. Inclusion of the photo-oxidation treatment process allows to obtain water with TOC levels down to 1 p.p.b. Photooxidative process is usually combined with recycling of treated water.
